SARS-CoV-2 Mitigations in Elevators
May 1, 2021

Identifying the characteristics of the coronavirus and elevator enclosure and examining the technical solutions for sanitizing the elevator
The discovery of SARS-CoV-2, the virus that causes COVID-19, in 2019, changed everyone’s lives, and solutions to return to normal are needed. One solution needed to reopen buildings and help restore business normalcy is elevator sanitization. Several systems have been designed, marketed and sold, but few have efficacy and testing studies approved by an authority. This article addresses third-party testing, governmental certification and known technologies being offered. It also considers the elevator space given the knowledge available today. To make informed decisions for purchase, owners should consider a method’s continuing efficacy, costs of deployment, long-term maintenance, whether there are any residual risks, and the ease of re-lamping, re-filtering and resupply. Elevator safety code concerns will also be considered.
Eliminating airborne pathogens (particularly SARS-CoV-2) inside an elevator with the technologies currently available is explored. Considering the elevator with forced or natural ventilation, with doors opening and closing, users entering and pushing buttons, coughing, sneezing, shedding the virus inside and outside the elevator, subjecting current and future passengers to the infectious viral droplets remaining airborne and landed viral particles (fomites), the complexity seems overwhelming. Eliminating the pathogens as quickly as possible, while not doing harm, is the goal. Not all the current technologies being marketed do this. Some do a little, making them better than others that do less. One technology, however, approaches perfection.
Virus Characteristics
Coronaviruses are a group of related RNA viruses that cause diseases in mammals and birds. In humans and birds, they cause respiratory tract infections that can range from mild to lethal. Mild illnesses in humans include some cases of the common cold, while more lethal varieties can cause severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and COVID-19. They are enveloped viruses with a positive-sense single-stranded RNA genome and a nucleocapsid of helical symmetry. According to Wikipedia, they have characteristic club-shaped spikes that project from their surface, which create an image reminiscent of the solar corona, from which their name derives, in electron micrographs. In relative-size analysis of negative stained SARS-CoV-2 (by electron microscopy), researchers have determined the diameter of this virus to range between 60 and 140 nm.[1] In addition to measuring the spherical size of the virus particle, it has also been confirmed that the size of the tumors surrounding the outermost surface of SARS-CoV-2 can vary in length from 9 to 12 nm.[2] Comparatively, a human hair has a typical thickness of 60-120 µm. So, 400-1,000 particles SARS-CoV-2 particles could make up the width of a hair.[2] Common colds are also caused by the coronaviridae group. Coronaviruses are not the cause of influenza A, which are in the orthomyxovirus group of viruses; ideally, all solutions can eliminate all disease-causing viruses.
The Centers for Disease Control and Prevention (CDC) published information regarding how the virus is transmitted. Infections with respiratory viruses are principally transmitted through the following modes:
- Contact transmission is infection spread through direct contact with an infectious person (e.g., touching during a handshake) or with an article or surface that has become contaminated. The latter is sometimes referred to as “fomite transmission.”
- Droplet transmission is infection spread through exposure to virus-containing respiratory droplets (i.e., larger and smaller droplets and particles) exhaled by an infectious person. Transmission is most likely to occur when someone is close to the infectious person, generally within about 6 ft.
- Airborne transmission is infection spread through exposure to virus-containing respiratory droplets comprised of smaller droplets and particles that can remain suspended in the air over long distances (usually greater than 6 ft) and time (typically hours).[4] Airborne transmission risk depends on the concentration of virus in the air in a room, the rate at which occupants in the space inhale the virus and the duration of exposure.[3]
According to the CDC, the epidemiology (study of the causes, distribution and control of disease in populations) of SARS-CoV-2 indicates most infections are spread through close contact, not airborne transmission. Diseases spread efficiently through airborne transmission tend to have high attack rates, because they can quickly reach and infect many people. We know that a significant proportion of SARS-CoV-2 infections (estimated at 40-45%) occur without symptoms and that infection can be spread by people showing no symptoms. Thus, were SARS-CoV-2 spread primarily through airborne transmission like measles, experts would expect to have observed considerably more rapid global spread of infection in early 2020 and higher percentages of prior infection measured by serosurveys.
Available data indicate SARS-CoV-2 has spread more like most other common respiratory viruses, primarily through respiratory droplet transmission within a short range (e.g., less than 6 ft). There is no evidence of efficient spread (i.e., routine, rapid spread) to people far away or who enter a space hours after an infectious person was there.[4]
Transmission of SARS-CoV-2 through air droplets indicates airborne exposure to the virus should be controlled. The American Society of Heating, Refrigeration, and Air-Conditioning Experts (ASHRAE) explains, “An infectious aerosol is a system of liquid or solid particles uniformly distributed in a finely divided state through a gas, usually air. (They are small and buoyant enough to behave much like a gas, yet they can be filtered out of the gas.)”[5] This would be the supply of the virus by an infected person talking, singing, yelling, coughing or sneezing. Changes to building operations, including the operation of heating, ventilating and air-conditioning systems, can reduce airborne exposures.
The air droplets, with the virus inside, are larger than the virus itself. The virus particle is very small: less than 140 nm. However, we know that it is larger and sticky due to the lipid envelope, as well as the sputum/saliva expelled in coughs/sneezes. Therefore, it clumps with other particles, making them larger.[6]
According to a recent study published in the New England Journal of Medicine, the virus has a survivability rate (how long before it is not hazardous without sanitization in the air and on surfaces) between several hours and several days. The study found the virus is viable for up to 72 h on plastics, 48 h on stainless steel, 24 h on cardboard and 4 h on copper. It is also detectable in the air for 3 h.[7] Another study indicates the virus environmental stability half-life is 1 h, meaning for a quantity of virus cells, in 1 h, half will have died in aerosol form.[1] Consideration of the time to inactivation must be a consideration in an elevator. Claims of viral inactivation efficacy by any manufacturer must only take credit for the inactivation of the living organism after subtracting the natural half-life inactivations.
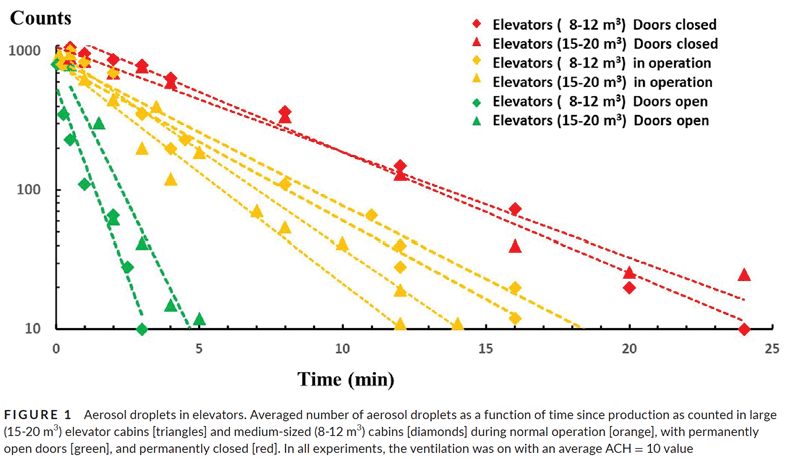
Elevator Information
The area of a passenger elevator is relatively small. For discussion purposes, let us say most cabins are 8 ft tall. A very large passenger elevator is 10 ft X 10 ft. = 100 ft2 area, giving an interior cubic area of approximately 800 ft3. A cab of more typical size in North America with a rated load of 2,500 lb is only 29.1 ft2 with a cubic area of approximately 233 ft3. These are the general parameters of air volume inside the elevator to consider for any discussions of airflow.
As a rule, elevators are engineered to move nonstop from the main landing to the top floor in 1 min or less. However, they can stop at every floor going up, leading to delays of approximately 10 s per stop. This is known as floor-to-floor time. A passenger can be in the elevator between 15 s from entry to exit on a one-floor run, up to 1 min on a nonstop to the top run, or a milk-run, stopping at every floor, which takes the time in the elevator to 1 min X (number of floors – 1) X 10 s. Therefore, a 25-floor rise can potentially keep a passenger in the elevator cab for at least 15 s to as long as 1 min + (24 X 10 s) = 5 min. The odds of this is unlikely; having a passenger demand at every floor is virtually nil. However, it is the non-theoretical maximum with one exiting passenger at each floor. Passenger exposure times in the elevator are typically 15 s-5 min.
Elevators are required by the ASME A17.1/CSA B44 Safety Code for Elevators and Escalators to have ventilation. Ventilation can be natural, meaning there may or may not be a ventilation fan. There must be ventilation openings equaling 3.5% of the area of the elevator. In our large elevator example, 3.5% of 100 ft2 = 0.35 ft2. The gaps around the doors, as well as the area of the fan cutout in the dome or top of the cab (if present) are included in this area calculation. Ventilation openings are required to be equally located in the upper and lower quadrants of the car enclosure.
As cabs move, air movement outside the elevator will naturally flow from the high-pressure side the low-pressure side of the car when moving. Some of this differential pressure will cause interior airflow, even without a ventilation fan. When not moving, only thermal currents generated by the heat of a passenger will cause airflow without a ventilation fan. The complexity of this internal airflow makes it impossible to claim where air currents will travel from/to or to calculate the mixing of air or where the airborne particles will go. Air will change in the interior of the elevator during movement and when the doors open and close, as studies that have modeled airflow demonstrate. All models make general assumptions to come to some conclusions, but the unique vent designs across all elevator manufacturers cannot have specific airflow patterns; they will be generalized.
Elevators have entrances at each landing, which, when open, will allow air movement from a building floor to the elevator (or vice versa, depending on the building pressurization design). The elevator hoistway and building pressure are likely never balanced, except coincidently; it is more likely that the elevator hoistway pressure will be slightly positive relative to the building floor pressure by design. There are many other influences: how windy the day is, the outside temperature relative to the inside temperature, how many elevators are in the group, whether the elevator doors are open or closed, if the elevator is moving or still and whether the building lobby has open/close doors or revolving doors. In the worst case, if a virus spreader were in a building lobby, and the air were to move into the elevator when the doors opened, elevator passengers would be exposed to that viral load for the duration of their ride (15 s-5 min). The converse is also true: if the spreader were in the elevator, and the air moves to the building, the lobby will be contaminated.
Details of a passenger in an elevator coughing and sneezing, with expected airflow using modeling to illustrate the dispersion patterns was written by T. Dbouk and D. Drikakis.[8] With every cough or sneeze being a potential virus spreader, elimination or inactivation of the virus based on this model can be visualized to lead to a sensible design consideration. The study concluded the simulations showed the risk of viral transmission inside the elevator was highest in elevators with the least air circulation. Their conclusion,“This is due to reduced flow mixing inside the elevator; regulatory authorities should thus define the minimum ventilation required depending on the type of building,”[8] recognizes the airborne nature of the virus. Additionally, if it is not blown away or inactivated in air, it remains accessible for breathing.
It is clear a mitigation inside the elevator is necessary to inactivate the virus. Simply venting from the cab into the hoistway, where the virus can survive for many hours, is not a solution. The outside of the elevator is in the same atmosphere, with air that has just moved from the cab. Filtering alone has residual risks, such as never changing the filter, removing the filter and not replacing it, discarding the filter when it may have active virus concentrations, etc. A form of treatment is, therefore, warranted.
Governmental Certification
The Environmental Protection Agency (EPA) has determined systems that make claims of viral inactivation systems must be tested and approved. In January, the EPA had only tested and certified systems utilizing triethylene glycol (TEG) for its efficacy and safety for human exposure and virus inactivation efficacy. This is an important change, given the technologies being introduced that make claims of virus inactivation without consumer protection. These technologies will and should come under more scrutiny, and buyers should be apprised of this fact. At the time of this writing, testing by the EPA or Food and Drug Administration (FDA) for the technologies explained herein to their efficacy and safety have not been published.
One manufacturer claims to have UL certification. While many UL standards are referenced in many codes, UL is an independent laboratory that writes standards and then tests for compliance to the standards. Without seeing the class the device was tested to, any claim must be carefully scrutinized. Complying to a fire and shock enclosure standard does not certify it will inactivate viral particles, for example.
Most jurisdictions adopt ASHRAE requirements. In a reaffirmed “Position Document on Infectious Aerosols,”[5] the organization concluded that, while its “Position Document on Filtration and Air Cleaning”[33] does not make a recommendation for or against the use of UV energy in air systems for minimizing the risks from infectious aerosols, the CDC has approved UV germicidal irradiation (UVGI) as an adjunct to filtration for the reduction of tuberculosis risk and has published a guideline on its application (CDC 2005, 2009) (Evidence Level A).” Other treatment technologies, including photocatalytic oxidation (PCO), photocatalytic electrochemical oxidation (PECO) and needlepoint bi-polar ionization (NBPI), were not even addressed.
From a governmental certification standpoint, EPA approval should be considered the gold standard for efficacy and safety for any viral inactivation system consideration in the elevator. Systems must demonstrate what process is used to inactivate the virus, how long the process takes, the rate of inactivation, any byproduct(s) and its process(es) and independent tests demonstrating their efficacy with an industrywide agreement for the test design. This would be, for example, a standard-sized elevator with typical ventilation openings and variant directional airflow. This would allow an apples-to-apples approach for determining the ideal solution for an elevator interior.
Virus Inactivation
How to inactivate (eliminate, kill, destroy) the SARS-CoV-2 virus includes several technological strategies: capture by filtering it and removal, bombarding its RNA with UV energies, using strong oxidants to destroy the protective shell surrounding the RNA or using viricides to inactivate the virus in a process known as “cell lysing.” Each method has degrees of efficacy depending on how it works, how it is deployed, long-term wearing of components and how it is maintained. Understanding how this is accomplished should be considered relative to health and safety, EPA certification, cost, efficacy and maintenance of the system. Getting the best results for the least amount of money can be accomplished, along with the understanding of what each method on the market promises, any negative health effects and the results that can be delivered.
Coronavirus outer membranes consist of protein stems connected by lipid (fat) cells forming a spheroid. The protein stems are like Velcro hooks that affix to cells in the human body. Once affixed, the virus invades normal cells in the human body. It replicates quickly, causing the body to launch immune cells to destroy the invader. The speed of virus replication is a primary problem of COVID-19, as immune-deficient people cannot develop immune protections as fast as or faster than the virus replicates. This leads to a cascade of the viral cells overwhelming normal cell function and preventing normal body function, particularly in the lungs. Being unable to absorb oxygen will lead to hospitalization, and, in the worst cases, to an intubation system utilizing a ventilator to force oxygen into the lungs to compensate for the oxygen transfer needed to live.
Any reduction of virus cell exposure will reduce risk of infection, but the things that destroy the virus may also destroy normal human cells. Therefore, any solution should be vetted by laboratory testing for certification and approval by governmental authorities or designed to prevent all exposure to humans. Those solutions are on “EPA List N” in its table of disinfectants.[10] Currently, only chemical solutions are on this list, and efforts to add other technologies are now being discussed due to the importance of efficacy claims and residual hazards of overexposure to ionized particles, ozone (O3) and/or other byproducts.
The cradle-to-grave installation of any technology must include consideration of the foreseeable risks of accidental exposure during installation while performing maintenance and when removing and disposing of the components. For example, high-efficiency particulate air (HEPA) filtering may be a strategy, but changing the filter can lead to accidental exposure to a significant amount of concentrated virus. Without a method to assure safe disposal of contaminated filters, there is a residual risk of accidental exposure. Another consideration is notification of lamps burning out. To believe there is full protection when a component is not working is more than a nuisance if the claim is viral inactivation.
Time is the most important consideration, as the passenger is in the elevator for at least 15 s and maybe up to 5 min. Any technology must inactivate the virus prior to inhalation in the best case. Assuming an aerosol droplet is sneezed, a study from the University of Amsterdam found the droplets took various times to fall to the floor of the elevator. This infers the droplets hang in the air for some period, accessible to be inhaled.[11]
Since the virus can live for up to two days, it can stay aerosolized for 3-25 min inside an elevator, and a passenger will be exposed for the duration of their occupancy. The ideal solution is one that can inactivate the virus in seconds, instead of minutes. The technology must be able to remove a significant number of viral particles in the shortest time possible, or it is not protecting as much as claiming protection. This is tantamount to a claim of a solution to kill bees, but it kills only 10 at a time out of a swarm of 100,000. The technology is proven to kill bees, but it is more appropriate for the occasional bee than a swarm.
Solutions
Eliminating viral particles in an elevator in the shortest time, without harming the passengers, is the goal. This assumes viral particles are present, the passengers are in the elevator with viral particles for 15 s to 5 min, an adult breathes 12-16 times a minute (preschool children up to 34 times a minute) and viral particles remain airborne for 25 min once shed from an infected person in the form of airborne droplets.
The solutions on the market are now discussed in this context. Each is followed with a conclusion as to its safety, maintenance requirements, efficacy based on studies, certification by testing organizations and governmental approvals. To keep this article general for normal reading, some science is necessary to understand the desired effects and any unintended effects of a technology.
Ionizers
A common solution in the market are ionizers. There are many variants of this technology — each with patented improvements over the various competitors — but all stem from the concept of generating ions. These devices are readily available and primarily used for indoor air quality (IAQ) management of particles floating in the air. They also attract viral particles and can produce ionized elements that destroy the viral membrane, inactivating the virus. The general technology is explained using descriptions by the various manufacturers, where referenced.
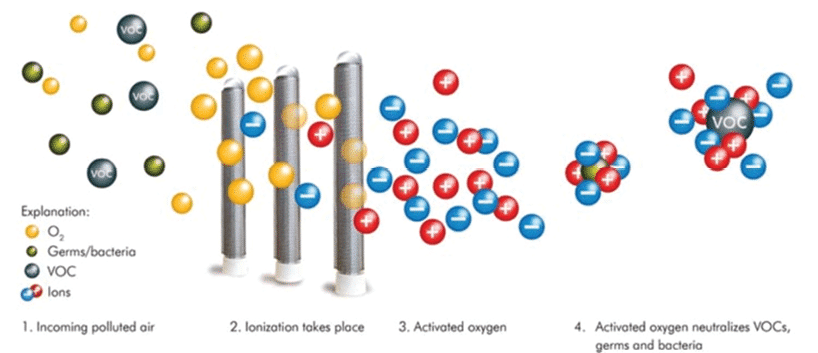
In physics and chemistry, plasma is a state of matter like gas in which particles are ionized. Heating a gas may ionize its molecules or atoms (reduce or increase the number of electrons in them), thus turning it into a plasma, which contains charged particles (positive and negative/ions).
Ionization technologies create a stream of ions that split passing water vapor into hydroxyls (OH ions) into a ventilation airstream. Due to the imbalanced charges, these ions are drawn back together to reform as H2O molecules, their natural state. During the transition, airborne viral particles may be attracted to the ions. They envelop SARS-CoV-2, for example, and puncture the protein spikes on its membrane, inactivating the virus. The primary inactivation of the virus is due to a molecular decomposition caused by the reactions of positive hydrogen and negative oxygen ions interacting with them.
Critics of these systems cite overblown efficacy claims of results. One study result indicates these ions can do lung damage.[13] These systems are typically installed in heating, ventilation and air-conditioning (HVAC) plenums and turned on when the fan motor is turned on, producing ions that react in the plenum creating agglomerations, particles growing in size due to the ions glomming onto the viral particle facilitating filtration.
In action, the plasma fields and numbers of produced radicals introduced into an airstream cannot inactivate all viral particles. Some particles bypass the plasma field; others may pass through the plasma field but are not attracted due to the limited number of radicals. While a reduction may occur, the process is not a complete inactivation. The ions only remain active for 1 min or less before restoring their non-ionized state.[14] This short life must be countered by producing many hydroxyl radicals and blowing particles through the plasma, the area bounded by ionized hydroxyls.
Ionizer technologies require creating a plenum on the elevator, a fan, a filter, and intake and discharge registers, where the cleaner air is forced into the breathable zone in the elevator through directed ducting. This type of ventilation system and retrofitting existing elevators would require costly labor and redesigned cab enclosures. The elevator sitting still for 30 min may experience a thorough sanitization. As the elevator begins moving people (the higher risk period), there is not enough time for significant sanitization to happen.
There have been no approvals by the EPA to determine efficacy of ionizer systems as an antimicrobial air treatment. While they are in common use in today’s building HVAC systems, they are not endorsed by ASHRAE. “Personalized ventilation systems, when coupled with localized or personalized exhaust devices, further enhance the overall ability to mitigate exposure in breathing zones, as seen from both experimental and computational fluid dynamics (CFD) studies in healthcare settings,” according to Yang, et al.; Bolashikov, et al.; and Bivolarova, et al.[15-20] ASHRAE follows up, “However, there are no known epidemiological studies that demonstrate a reduction in infectious disease transmission. (Evidence Level B)”.[33]
Many manufacturers of ionizing systems make claims that their system is an antimicrobial air treatment. However, none have published any EPA-reviewed test results for SARS-CoV-2 or its approved surrogate MS2 phage inactivation.[21] Their primary uses to date are improving IAQ at slow rates. Manufacturer claims of killing viral particles may be true in that the technology does inactivate them; however, the speed of inactivation and the complexity of distributing “cleaned” air is the issue.
Noncatalytic ionizer systems go by the following names: bi-polar ionization (cold plasma ionization) and NBPI. These systems produce negative and positive ions when electricity is applied to a tube with two electrodes that react with water vapor and oxygen in the air to create free radicals. The free radicals can kill microorganisms and break down odors, improving IAQ.[22] Note that this claim does not include viral reduction at any significant rate in a short time. Early systems also developed O3, but modern systems produce less O3 to comply with EPA exposure limits. One manufacturer tested in accordance with UL 867, which limits O3 to 0.05 parts per million by volume.
NBPI
NBPI that has been independently tested on viral particles by Innovative Bioanalysis, a laboratory in Costa Mesa, California, showed a 99.4% inactivation of viral particles at 30 min in a chamber under conditions intended to replicate an occupied building.[23] While these results seem impressive, testing was done in a 1-ft3 chamber with unlimited ions without exterior ventilation. This does not consider the airflow of the interior of an elevator. The results also did not isolate the natural dying of the viral media and calculate the actual ionic inactivation efficacy. For example, if 100,000 virus particles were introduced, an estimated 7% will die naturally, leaving 93% activated organisms. When claiming efficacy, reports must indicate the efficacy on the living 93%, not taking credit for the 7% natural decay that occurs without any treatment. The inactivation also took minutes to inactivate the virus at high concentrations, leaving this methodology ineffective for use in an elevator.
Otis recently announced an air purification system claiming its modeling results indicated that the use of NPBI reduced risk exposure 20-30%, depending on the ride time and riders’ positions within the elevator. Furthermore, the use of NPBI, combined with proper mask usage by all passengers, yielded a 60-65% reduction in relative risk.[24] This result is positive but not a sanitizing solution in the timeframe of a normal elevator ride. Ideally, reduction of viral particles in seconds would be a preferable solution.
Catalytic Ionizers
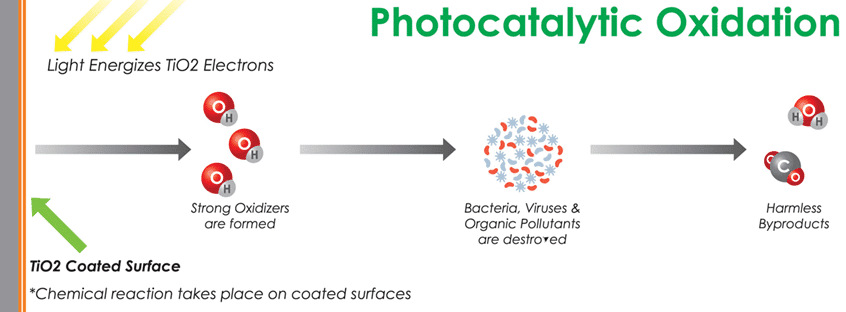
PCO and PECO
UV light is applied in photocatalytic processes to excite and activate a catalyst to begin the chemical reaction adding more oxidizer radicals to the air (in addition to the UV light itself). PCO air systems use a very energetic band of light, UV-C, to initiate the reaction. However, UV-C is also known to produce O3, which is also a potent oxidizer and toxic to living things. Caution should always be used to ensure no people, pets or houseplants are exposed to elevated levels of O3.[25]
The most potent oxidizer radicals are hydroxyls (OH-) and can oxidize even the toughest organic molecules with the help of water and oxygen in the air. Oxidation generally converts organic compounds into carbon dioxide, water and other trace gases. This air purification process consists of a pure or doped metal-oxide semiconductor material such as titanium dioxide as a catalyst activated by a UV light source, either UV-A (400-315 nm), UV-C (280-200 nm) or UV-V (under 200 nm) to generate OH- ions. O3 is also generated, unless the UV-C is limited to 253.7 nm, because oxygen is not absorbed in the PCO process at that frequency.
Studies[26] have documented other byproducts of PCO, including formaldehyde, a known carcinogen. Additionally, it can produce other undesirable compounds such as trichrloethylene, acetone, benzene and toluene.[28]
Haldane King writes in “PECO v. PCO Air Purifiers: How are they different?”:
“The major difference between the PCO and the PECO processes is the consideration of the negative electrons and positive holes after their generation by the photons. Since negative electrons tend to quickly recombine with the positive holes, fewer holes are available to react in the case of the catalysts used in PCO, which results in low quantum efficiency. On the other hand, the PECO process binds the free electrons and keeps them separated from the positive holes long enough for the holes to form the hydroxyl free radicals.
“This fundamental difference between the two processes [has] major consequences. Due to the recombination of electrons and holes, the PCO process can utilize only a small fraction of the photons, which makes the overall process inefficient. A potential danger of the inefficient process is that some systems may yield toxic oxidation byproducts, such as formaldehyde, due to an incomplete reaction. PECO, on the other hand, has a quantum efficiency many orders of magnitude higher than that of the PCO process, which makes the PECO oxidation process extremely fast, resulting in complete oxidation with no byproducts.”[25]
While PCO and PECO may create radicals, they require airflow slow enough to inactivate viral particles before the cleaned air reenters the elevator. No studies demonstrate this rate of viral inactivation, nor has the EPA approved this as an antimicrobial air treatment using the PECO process.
KLEEMANN advertises a PCO ventilation system that claims to remove 99.76% of influenza virus but does not provide studies to justify this efficacy claim. While it may be true that viral particles exposed to PCO sterilization will be inactivated, there is no study provided to determine if all viral particles that enter the system are inactivated, nor how long this takes, on the company’s website.[29] It is true that the radicals can inactivate the virus, but how many, how quickly and how effectively in an elevator need further testing and certification to assure safety and verify efficacy.
Photohydroionization (PHI)
This oxidation process utilizes dual-wavelength UV elements targeted onto a multi-metal catalyst surface coated with titanium dioxide, silver, copper, rhodium and hydrophilic coating. UV energy at 254 nm strikes the target surface, activating the production of hydroxyl radicals, super oxide ions, and hydroperoxides on the PHI cell surface. The hydrophilic surface absorbs water in the air, then splits them into OH radicals in the advanced oxidation process.[29] This technology uses a second lamp emitting UV energy at 185 nm. The photon energy at this wavelength is sufficient to split oxygen molecules. O3 molecules are reduced back to oxygen by the 254-nm UV energy that is also emitted from the UV element. O3 emission results are in the .01 ppm range — well below 0.05 ppm, which the FDA and California consider safety limits.[30]
Old Coast Heating + Air Conditioning explains in “What is Photohydroionization and How Does it Improve IAQ?”:
“PHI is a patented ionized hydroperoxide technology developed by RGF Environmental in the late 1990s. In simple terms, PHI cleans indoor air by changing the chemical properties of particles in the air using a very fine mist of hydrogen peroxide. The technology behind PHI is actually pretty simple. It simulates natural hydroperoxides in the outdoor air — Mother Nature’s own system for improving IAQ. To do this, PHI uses a rare metal catalyst and a hydrating agent activated by a broad-spectrum UV light. The tools react with ambient moisture in the air to create hydroperoxides. The hydroperoxides are then ionized to make PHI even more effective.”[31]
While PHI may create radicals, they, again, require airflow slow enough to inactivate viral particles before the return air reenters the elevator. No studies demonstrate this rate of inactivation, nor has the EPA approved this as an antiviral mitigation technology. It is not suitable for elevators because of the time delay to virus inactivation, installation of filtered ventilation required and exposure time of passengers. These are systems designed for building HVAC systems to remove particles at a slow and steady rate but cannot meet the challenges of viral inactivation in the short time in which a passenger in an elevator is exposed.
UVGI
UVGI is a disinfection method that uses short-wavelength UV (UV-C) light to kill or inactivate microorganisms by destroying nucleic acids and disrupting their DNA or RNA, leaving them unable to perform vital cellular functions. The entire UV spectrum can kill or inactivate many microorganism species, preventing them from replicating. UV-C energy at 253.7 nm provides the most germicidal effect.
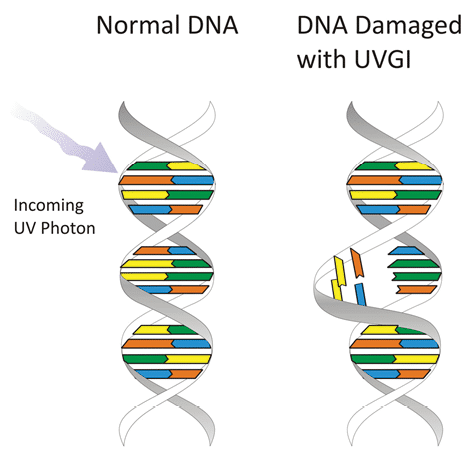
The Bumbling Biochemist has a simple explanation of UV RNA destruction:
“Our cells use DNA to hold our genetic blueprint (genome) — containing instructions for making (and regulating) all the proteins, etc. we need to live. You don’t want to mess with it, because, if you do, things like cancer can occur. SARS-CoV-2. . . is an RNA virus — it has an RNA genome, instead of a DNA one – and it’s single-stranded, unlike our genome, which is double-stranded.
“DNA & RNA nucleotides structures correspond to light in the UV range. When DNA or RNA absorbs UV light, it can break bonds to form improper bonds, like strong bonds called pyrimidine dimers between neighboring bases in the same strand. If these don’t get fixed, or if they get incorrectly ‘fixed’ before the genome gets copied, there will be permanent mutations in all future daughter cells.
“Our cells have complex machinery on guard to try to correct errors before the DNA gets copied and the error gets passed on, but if they get too much UV exposure, they get overwhelmed, and errors slip through the cracks. And, if those mutations do things like mess up a regulatory molecule, cells can start growing uncontrolledly to form a cancerous tumor.
“The virus has no hope of fixing this broken RNA, because [it lacks] the complex machinery of DNA, and without functioning RNA, it can’t survive. UV-C light can be used as a disinfectant — BUT for SURFACES (and maybe air) – NOT for people. If the virus is on someone’s skin, and you shine them with UV light, you might kill the virus on the skin, but you’re also putting that person at risk of skin cancer. And, if the virus is inside someone (they’re already infected) — shining UV light on them will not cure them — the UV light [will] get absorbed by their skin (potentially messing up their DNA) before it had any chance of even getting to the virus to kill it.”[32]
UVGI degrades organic material and inactivates microorganisms at the DNA level. The system is not a filter; thus, inactive particles remain in the airstream, which, in the case of dead fungal spores, may still cause a negative human response to their integral mycotoxins. The most effective wavelength range for inactivation of microorganisms is 220-300 nm, with peak effectiveness near 265 nm. The typical source of UV-C in commercial and residential air and water systems is low-pressure mercury-vapor lamps, which emit, mainly, near-optimal 253.7 nm. UV-C systems may be installed inside HVAC systems, irradiate air near the ceiling or be incorporated in a standalone/portable air cleaner.[33]
The time to inactivate viral RNA requires either remaining in the UV light for a longer duration (slower airflow) or increasing the intensity of the UV light to inactivate faster (higher airflow). These variables need to be considered when designing this system.
One manufacturer claims to have UL certification; however, the company was not evaluated by the EPA to determine if human exposure levels or efficacy were considered. The claim is that UL performed a study[34] comparing the levels of various dusts with particulate matter sizes (in units of particle mass [PM]) of PM10 and PM2.5, volatile organic compounds (VOCs) and carbon dioxide. The following took place in two elevators, both of which were being actively used by people in an occupied building.
- In a duplex group, one elevator (the control) has a standard exhaust fan.
- In the other elevator (the “test”), there was a Sterilyft installation.
- The particulate levels in the analyses were monitored continuously for a month.
While these analyses are relevant to air quality, they are not directly relevant to efficacy against SARS-CoV-2. The cover letter tries to link the particulate size difference in PM10 and PM2.5 measurements between the control and test elevators to a reduction in viral particles, while also utilizing a UV light system for viral inactivation. The UL study did not measure virus levels, making the efficacy essentially invisible. The sample size is small, and there is no information to characterize whether the control and test elevators were similar, in terms of loading of passengers, hours of use or other important factors. This study has very little value in characterizing the efficacy of Sterilyft against airborne pathogens. It likely does inactivate some virus particles, but how many and how quickly?
Without seeing the class the device was tested to, any claim must be carefully scrutinized. Complying to a fire and shock enclosure standard does not certify it will inactivate viral particles.
One manufacturer, the product of which is sold under the name “Sterilyft,” has produced UL test results for evaluation. This study[34] has more to do with IAQ with claims of viral inactivation efficacy without providing measured results. For example, it doesn’t give time data for how long it took for car 12 (with Sterilfyt) to reduce particles lower than those in car 13 (control/without Sterilyft), it only says the air monitoring study was conducted over the course of a month (July 20-August 20, 2020) in the cars 12 and 13. A careful review of this study reveals some anomalies.
PM10 and PM2.5 (noted in the study’s “Table after Attachment C”) shows the data over the entire month. From the minute it started, car 12 already had many fewer particulates than car 13 did. One might have expected to see data of similar particulate densities a few days prior to treatment to determine if both elevators had similar air quality, instead of assuming they would be the same (as this data implies). If car 12 was already lower, how effective was the treatment, relative to natural particulate differences? Additionally, an unexplained gap in the data on August 8-9 in car 12 is followed by an increase in particulates that still doesn’t go as high as the number of particulates in car 13. Compared to the graph on page 11 with the same timestamps, the data missing from the table is in the graph. This implies there are innately fewer particulates in car 12.
This technology is not suitable for elevators because of the time delay to virus inactivation, installation of filtered ventilation required and exposure time of passengers. This system is designed for building HVAC systems to remove particles at a slow and steady rate but cannot meet the challenges of viral inactivation in the short time in which a passenger in an elevator is exposed.
Dry Hydrogen Peroxide (DHP)
Another technology uses a proprietary photocatalyst to produce DHP. The photocatalyst is applied to a framed polyester fiber mesh, which is placed within a regulated airflow. Upstream of the photocatalyst-coated mesh is a UV-A bulb centered on 363 nm. An electronic ballast housed within the device casing draws standard voltage and uses it to power the bulb.[36]
The DHP produced mixes homogeneously in the air, attaching to viral particles, destroying the membrane, rendering it inactive. This has been studied with positive effects in hospitals. The results of this study at Unidad Nacional de Oncologia Pediatrica demonstrated that DHP was effective in reducing the residual microbial bioburden on surfaces and in the air, though reductions in the air did not reach statistical significance.[37]
This technology is not suitable for elevators because of the time delay to virus inactivation, the installation of filtered ventilation required, its lack of airborne viral particle reduction and exposure time of passengers. This system is designed for building HVAC systems to remove particles at a slow and steady rate but cannot meet the challenges of viral inactivation in the short time an elevator passenger is exposed.
Plasma Cluster Ions
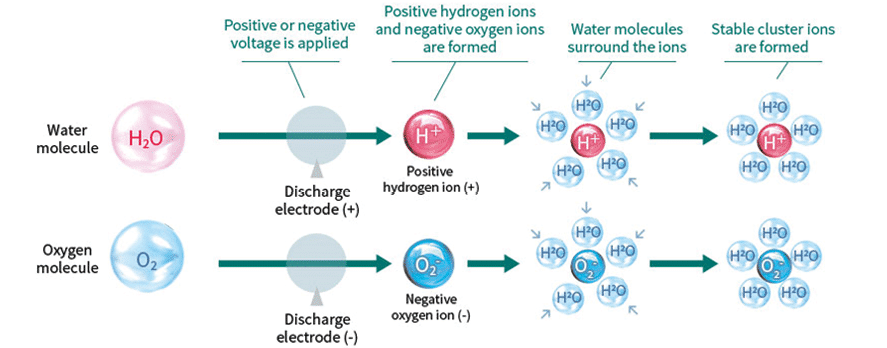
This technology uses plasma discharge, in which voltage is applied to a discharge electrode, creating positive hydrogen and negative oxygen ions generated from the water and oxygen in the air. The ions attract water molecules in the air. These surround the ions and form stable cluster ions. The ions bond on the surface of airborne viruses and other substances and change into OH radicals. With their extremely strong oxidizing power, the OH radicals quickly extract hydrogen from the protein on the surface of viruses and other substances, thus decomposing the protein and suppressing activity. The surface of things like bacteria and allergens consists mainly of protein. Removing the hydrogen atom (H) from this structure inactivates the undesirable substance. Furthermore, the OH radical bonds with the removed hydrogen atom (H) to immediately form water (H₂O), which is returned to the air.[38-39]
This process was shown in two large studies in 2009[40] and 2015[41] to be effective against H1N1 and H7N9 viruses. Inactivation of 99.9% of the virus took 2 h in a 1-m3 box, again not simulating an elevator.
This technology is not suitable for elevators because of the time delay to virus inactivation, installation of filtered ventilation required and exposure time of passengers. This system is designed for building HVAC systems to remove particles in a slow and steady rate but cannot meet the challenges of viral inactivation in the short time a passenger in an elevator is exposed.
Vapors
Vaporized Hydrogen Peroxide (VHP)
A common sanitizing technology involves using vaporized liquid hydrogen peroxide, the vapor of which fills the space to disinfect all exposed surfaces. The space must be unoccupied during VHP treatment due to its toxicity. It requires spaces, including all doorways, plumbing/electrical penetrations and HVAC supply and return vents to be sealed to prevent vapor from escaping. After prescribed exposure times, remaining H2O2 vapor is scrubbed and converted back to oxygen and water before the space can be safely reoccupied. The effectiveness and safety of VHP when generated inside active HVAC ducts and occupied spaces has not been rigorously studied. VHP is, however, hazardous at high concentrations, and lengthy exposure is often necessary to inactivate bacteria and viruses in sealed spaces.
This technology is not appropriate for an elevator, because people are present randomly. This is unlike a room that can be predictably emptied.
Filtering
HEPA Filtering
HEPA filters have been used for decades. The U.S. Department of Energy and EPA define HEPA based on a minimum efficiency of 99.97% when tested with an aerosol of 300 nm in diameter. The EPA defines the diameter of 300 nm as the “most penetrating particle size” (MPPS). However, the MPPS can vary around 0.3 μm, with the precise value depending on the nature of the aerosol particles, type of filter material, and flow rate.
The World Health Organization (WHO) recommends filtration levels for patients who require airborne isolation precautions should be placed in an airborne precaution room.[42] An airborne precaution room is one with more than 12 air changes per hour (ACH) (e.g., equivalent to more than 80 l/s for a 4 X 2 X 3 m3 room) and controlled direction of airflow and can be used to contain airborne infections.[42] A mechanically ventilated room is equivalent to the airborne infection isolation room described by the CDC, which the organization says should have special features in air handling and airflow direction, including:
- A negative pressure differential exceeding 2.5 Pa
- An airflow differential exceeding 125 cfm (56 l/s) exhaust versus supply
- Clean-to-dirty airflow
- Sealing of the room, allowing approximately 0.5 ft2 of leakage
- More than 12 ACH for a new building and more than 6 ACH for existing buildings (e.g., equivalent to more than 40 l/s for a 4 X 2 X 3 m3 room) for an old building
- An exhaust to the outside, or a HEPA filter if room air is recirculated.
Using this as a design guide, getting the elevator enclosure to this level can be achieved. However, discussion regarding whether it is necessary to filter to this level is needed.
The diameter of the SARS-CoV-2 microbe is approximately 100 nm and further wrapped in saliva, making particles as large as 140 nm. Thus, HEPA filtering alone is apparently not adequate. Another mechanism must be provided to make particles electrostatically stick to the filter media, and/or some other inactivation technology should be used with the filter.
Designing any airflow system entails determining where the clean air should be. In the elevator, for instance, this is in the breathing zone. Next to be considered is the exchange rate: An adult breathes 12-16 times a minute, and preschool children breathe up to 34 times a minute.[43] Changing the air can be easily visualized by thinking you are outside when the wind blows 1 mph (5,280 ft/m). An adult breathing 16 times a minute has 5,280/16 = 330 ft of air pass by between breaths. Fresh air is simply never rebreathing exhaled air or air exhaled by others, because the wind blows. When in a room, air changes due to ventilation velocity, and cubic-area calculations can be made. For example, if a 100-cfm system is designed in a 30-ft3 area, after all restrictions (like the filter) are accounted for, there are 3.3 air changes per minute and 100/30 = 3.3 X 60 = 198 ACH. This far exceeds the WHO recommendation of more than 12 ACH. Because of the box nature of the elevator and the location of code-required ventilation holes and intake and exhaust ports, it is likely that not every air particle is exchanged, justifying a higher ACH rate.
However, a smart design would ensure that the breathing zone (2-6 ft) is where the filtered air goes and not consider exchanging air near the floor. Complexity is added when the doors open and new potential viral shedders enter: their talking, coughing and sneezing will introduce viral particles into the space with the need to exhaust and filter them. In any scenario, ventilation/filtering systems alone with a high ACH can remove all particles in the time of an elevator ride.
Any ventilation designs would have to be incorporated into the cab enclosure, and only a few have been introduced to the elevator inventory. Any designs would require substantial labor to install, a redesign of cab interiors and ongoing maintenance for the life of the ventilations system. Also, it cannot be forgotten that used filters potentially contain live virus and must be disposed of safely, a task the elevator industry is not prepared for without training.
This process is suitable for elevators, because of the time to filter and ensure clean air is provided rapidly in the breathing zone. Where other technologies, such as UV, PCA, PECO and NBPI are incorporated, there will be further reduction in viral particles. However, this process has the most cost due to the intensive installation on the elevator.
Aerosols
Aerosolized Viricides
The antimicrobial properties of TEG were studied in 1943 at the University of Chicago.[44] The studies showed the amphiphilic properties that render viruses inactive when aerosolized TEG microdroplets condense on the membrane surface of the airborne virus.[45]
In January 2021, the EPA issued a landmark approval for Grignard Pure as an aerosolized antiviral air treatment product specifically for SARS-CoV-2 in the fight against COVID-19. This product is released continuously into the air of indoor spaces at low concentrations that effectively reduce the level of airborne virus particles. The manufacturer claims to effectively inactivate more than 98% of an airborne surrogate virus in 30 s at a non-visible haze level.
A report of extensive testing, “Assessing the Safety of Grignard Pure” by researchers Dr. Jack Caravanos of New York University and retired EPA scientist William Jordan, now an independent environmental consultant, evaluated the ingredient TEG in February 2021. To summarize its conclusions:
- The EPA and FDA have judged TEG to be “low risk” when used in products regulated by the agencies since the 1960s.
- Inhalation exposure to TEG from the use of Grignard Pure would be below recommended safe exposure limits and hundreds of times lower than the concentration that caused no systemic effects in inhalation toxicity studies with laboratory animals.
- Exposure to TEG aerosol may cause transient, mild irritation of eyes, nose or throat in some sensitive individuals.
There have been no approvals by the EPA to determine efficacy of ionizer systems as an antimicrobial air treatment. While they are in common use in today’s building HVAC systems, they are not endorsed by ASHRAE.
MS2 bacteriophage, an EPA-approved surrogate for SARS-CoV-2 and dozens of pathogens including influenza, was used for the testing. The surrogate virus is harder to inactivate than SARS-CoV-2, according to the EPA’s hierarchy of microorganisms. Testing indicates more inactivation of airborne virus particles in 5 s, not including natural die-off, exceeding 95%. This is being put to scientific scrutiny under the guidance of Dr. Gediminas Mainelis of the department of environmental sciences at Rutgers and Dr. Gurumurthy Ramachandran at the department of environmental health and engineering at Johns Hopkins University.[45] It is expected that the quick inactivation rate will reduce the load of infectious viral particles in the air.[46]
Support for this new study comes from a real-life test in the New Amsterdam Theater in NYC was undertaken by Jaros, Baum & Bolles (JB&B). The system aerosolizes a very small amount of liquid TEG on a hot plate, then blows the particles into the HVAC airflow. Feedback sensors measure TEG density and feeds back commands to deploy more product to maintain a minimum level ensuring viral inactivation level exceeding 99%. The test results exceeded all expectation for efficacy.
JB&B reported the microdroplet aerosolized airborne antimicrobial was proven to be delivered effectively, reliably and consistently to all spaces served by that HVAC system. It was demonstrated that, in a microdroplet state, TEG effectively behaved like a gas in its ability to distribute, dilute and disperse into occupied spaces. In real time, product concentration in the occupied spaces was reliably measured, and feedback control loops were proven to precisely control the output from the atomizer machines.
This result is important, as it meets the time criterion for use in an elevator timeframe, but the following questions arise:
- How does it replenish?
- Are there health concerns?
- Where did this come from?
- When was this discovered, and why did it take a year to get this approval?
One sector in which Grignard operates is stage fogging (the white wisps of fog that drape the feet of the actors for dramatic effect). Decades ago, the discovery of microbial colonies growing in the reservoirs of the ingredients used to generate the fog led to research into what would eliminate that problem. In experiments to provide a nontoxic, yet equally effective, fog mist, Grignard discovered TEG would prevent bacterial growth.
We all have likely been in a room with TEG fogging systems. TEG for fogging machines is widely available for home use.[48] Fast-forward to 2019: tests were done that discovered TEG also inactivates SARS-CoV-2 within 30 s of contact. This was brought to the attention of the U.S. Coronavirus Task Force, and accelerated testing was done. The testing culminated in the first EPA antiviral air-treatment approval in history.
Studies of TEG in the 1940s[45] and previous research files explain the EPA and FDA’s judgment of the active ingredient in TEG to be “low risk.” The following appears in a 2021 approval of emergency exemption for air treatment, referencing a 2003 EPA reregistration eligibility decision (RED):
“The [EPA] has completed its human health and environmental review for [TEG] and is issuing its risk management decision. The agency has decided that [TEG] is eligible for reregistration. This RED addresses the use of TEG as a bacteriostat (against odor-causing bacteria) for air sanitization and deodorization. This RED reassesses the exemption from the requirement for a tolerance for these uses. This tolerance exemption is listed in 40 CFR 180.920 (69 FR 23124, Apr. 28, 2004)” [46]
TEG is also approved by the FDA as a preservative for food packaging adhesives as listed in 21 CFR 175.105. Currently, however, there are no EPA-registered products for this use. TEG also has an indirect food additive regulation (21 CFR 177.1200 [4/1/04]) for its use as a plasticizer in cellophane. This use is regulated by the FDA.[49]
An EPA reregistration of TEG in 2006 concluded that, from the available animal studies and other data, TEG exhibits low toxicity. Additionally, there is a reasonable certainty of no harm to the general population. This includes study of infants and children having aggregate exposures to TEG (as either an active or inert ingredient), including all anticipated dietary (food and water) exposures and all other types of exposures for which there is reliable information.[49]
The manufacturer claims aerosolized TEG inactivates airborne viruses at a level invisible to the human eye.[51] This is an important point in that, with decades of approval to use the much denser TEG fogger, the air-sanitization-treatment version is invisible yet retains its viral inactivation characteristics at densities hundreds of times less than the level considered safe by the EPA and FDA.
Lastly, only a minute amount of TEG is required. By some estimates, 1 gal. will last a typical elevator eight weeks. Devices requiring no ventilation; filtering; or modification, except for adding a reservoir and power to the misting device, are being designed for elevator use.
This process is suitable for elevators, because of the time to inactivation is immediate when the density of aerosolized particles are maintained via feedback. Newly introduced viral particles, when the doors open, would encounter the inactivation viricide immediately. There are no toxic residuals; vacuuming inactivated particles is no different than current janitorial practices. Feedback detectors ensure efficacy will remain high even when dilution occurs when doors open.
This system meets all conditions for use in an elevator. It must be considered that typical forced ventilation will cause a continued dilution into the hoistway enclosure, however. At some point, the entire area would also be sanitized. Risk assessment should consider the effects of excess TEG on unmonitored hoistway and building spaces. Questions such as, “How long can it remain airborne?” and “What do the TEG molecules decay into after years of sitting on ledges of elevator hoistway components?” should be posed.
ASME A17.1/CSA B44 Code Considerations
Currently, the A17.1/CSA B44 Safety Code for Elevators and Escalators does not address sanitization devices for use on elevators. HVAC devices have specific requirements when the elevator is outside with transparent enclosures but no specific requirements for temperature control, filtering or sanitizing.
Any devices installed in an elevator will require compliance with flame-spread and smoke-generation requirements in the event vandals attempt to set fire to interior components (an all-too-common occurrence). These are the typical requirements that apply to any cab components added to the elevator interior.
Possible considerations for the A17.1 Standards Committee should include requiring EPA approval for devices installed for permanently affixed sanitizing purposes, O3 limits, minimum ACH for filtration systems, perhaps setting PM limits where filtration claims can be tested and maintenance control program maintenance requirements for these devices.
These are not traditional elevator design requirements, however, as technological solutions are to be installed in the elevator, integrating them into car operating panels, car power requirements, new access panels, new intake and exhaust registers and anti-vandalism mitigations are a few of the design considerations that should be deliberated. Language should also be considered for protection from toxic substances, if only to reference EPA, NIOHS, and FDA requirements for the technologies employed as applicable.
Conclusion
This article set out to identify the characteristics of the virus and elevator enclosure, and the technical solutions to sanitize the elevator. It also set out to determine the ideal technological solution and evaluate the known solutions available. This is based on several metrics, efficacy of viral inactivation demonstrated in testing, governmental review and approval, ease of installation and maintenance, and continued efficacy as the systems age.
The benefits of the viricidal aerosol approved by the EPA far outweigh those of other technologies because of its very fast inactivation of virus particles without negative effects to humans. Having decades of use in public areas as a fogger at thousands of times the density of the viricidal density needed in the elevator makes the strongest case for using this system safely.
Considering the time of flight for passengers and the immediacy of being exposed to other passengers who may be shedding viral particles requires the most immediate inactivation. Complexities of airflow can be ignored, given feedback from the sensor to provide resupply in the air. Positioning of the sensor to ensure a correct representation of the density should be studied further, and airflow studies incorporating forced ventilation and natural ventilation should be done.
References
[1] Bar-On et al.. “SARS-CoV-2 (COVID-19 by the numbers)” (www.ncbi.nlm.nih.gov/pmc/articles/PMC7224694/#__ffn_sectitle) (2020).
[2] Cuffari. “The Size of SARS-CoV-2 and Its Implications” (www.news-medical.net/health/The-Size-of-SARS-CoV-2-Compared-to-Other-Things.aspx).
[3] Buonanno et al. “Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection” (www.sciencedirect.com/science/article/pii/S0160412020320675) (2020).
[4] CDC. (www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html).
[5] ASHRAE. “Position Document on Infectious Aerosols” (www.ashrae.org/about/position-documents) (2020).
[6] P. Amnizi, B. Stephens. “HVAC filtration for controlling infectious airborne disease transmission in indoor environments: Predicting risk reductions and operational costs.” Building and environment Vol. 70 (2013): p. 150-160 (doi.org/10.1016/j.buildenv.2013.08.025).
[7] Machamer. “How long can the virus that causes COVID-19 live on surfaces?” (hub.jhu.edu/2020/03/20/sars-cov-2-survive-on-surfaces) (2020).
[8] T. Dbouk, D. Drikakis. “On airborne virus transmission in elevators and confined spaces,” Phys. Fluids 33, 011905 (doi.org/10.1063/5.0038180) (2021).
[9] ASHRAE. “Position Document on Filtration and Air Cleaning” (www.ashrae.org/about/position-documents) (2018).
[10] EPA. “List N Disinfectant Results Table” (cfpub.epa.gov/giwiz/disinfectants/index.cfm) (2021).
[11] van Rijn et al. “Reducing aerosol transmission of SARS-CoV-2 in hospital elevators” (doi.org/10.1111/ina.12744) (2020).
[12] Healthline. “Breathing Rates” (www.healthline.com/health/normal-respiratory-rate#normal-rate-in-adults) (2021).
[13] Ionizer Facts. (ionizer-facts.com).
[14] NOAI. “Ozone vs Hydroxyl Generators” (www.noai.org/ozone-vs-hydroxyl-generators) (2020).
[15] J. Yang, C. Sekhar, D. Cheong Kok Wai and B. Raphael. “CFD study and evaluation of different personalized exhaust devices,” HVAC&R Research 19(8):934–46 (2013).
[16] J. Yang, C. Sekhar, D. Cheong and B. Raphael. “Performance evaluation of an integrated personalized ventilation-personalized exhaust system in conjunction with two background ventilation systems,” Building and Environment 78:103–10 (doi.org/10.1016/ j.buildenv.2014.04.015) (2014).
[17] J. Yang, S.C. Sekhar, K.W. Cheong and B. Raphael. “Performance evaluation of a novel personalized ventilation-personalized exhaust system for airborne infection control,” Indoor Air 25(2):176–87. (DOI.org/10.1111/ina.12127) (2015).
[18] J. Yang, C. Sekhar, D.K.W. Cheong and B. Raphael. “A time-based analysis of the personalized exhaust system for airborne infection control in healthcare settings,” Science and Technology for the Built Environment 21(2):172–78. (DOI.org/10.1080/10789669.2014.976511) (2015).
[19] Zhecho D. Bolashikov, Maria Barova and Arsen K. Melikov. “Wearable personal exhaust ventilation: Improved indoor air quality and reduced exposure to air exhaled from a sick doctor,” Science and Technology for the Built Environment, 21:8, 1117-1125 (doi.org/10.1080/23744731.2015.1091270) (2015).
[20] Mariya P. Bivolarova, Arsen K.Melikov, Chiyomi Mizutani, Kanji Kajiwara and Zhecho D.Bolashikova. “Bed-integrated local exhaust ventilation system combined with local air cleaning for improved IAQ in hospital patient rooms,” Building and Environment, Volume 100, p. 10-18, ISSN 0360-1323, (doi.org/10.1016/j.buildenv.2016.02.006) (2016).
[21] Rutgers. “MS2 Surrogate to Covid 19 Testing.”
[22] ASHRAE. “Standard 62 IAQ Procedure: Reduced Outdoor Air for Auditorium,” ASHRAE Journal (Vol. 48, May 2006)
[23] FSG. “How Bipolar Ionization Inactivates Airborne Pathogens and Viruses in Facilities,” (fsg.com/bipolar-ionization-inactivate-pathogens-viruses-facilities) (2020).
[24] Otis. “Air purification in elevators today” (www.otis.com/documents/256045/28643078/OTS-15549_TechPaper_R7.pdf) (2020).
[25] Haldane King. “PECO v. PCO Air Purifiers: How are they different?” Molekule Blog (molekule.science/%C2%ADpeco-v-pco-air-purifiers-how-are-they-different) (2019).
[26] Lexuan Zhong and Fariborz Haghighat. “Photocatalytic air cleaners and materials technologies – Abilities and limitations,” Building and Environment, 2015; 91: 191 (DOI.org/10.1016/j.buildenv.2015.01.033).
[27] EPA. “Cost Analysis of Activated Carbon vs. Photocatalytic Oxidation for Removing VOCs from Indoor Air 90503A,” EPA Number: EPA/600/J-98/298.
[28] Aplinkos Tyrimai and Inžinerija ir Vadyba. “Photocatalytic Oxidation Performance to Removal of VOCs in Indoor Environment,” Environmental Research, Engineering and Management, No. 4(58), p. 27-33 ISSN 2029-2139 (erem.ktu.lt) (2011).
[29] KLEEMANN. “Elevator Air Purifier” (kleemannlifts.com/content/elevator-air-purifier).
[30] PHI. (www.environmental-expert.com/news/photo-hydro-ionization-665721/full-article) (2021).
[31] Old Coast Heating + Air Conditioning. “What is Photohydroionization and How Does it Improve IAQ?” (oldcoasthvac.com/what-is-photohydroionization-and-how-does-it-improve-iaq) (2021).
[32] The Bumbling Biochemist. “UV light – what happens when it and DNA or RNA get into a fight…” (thebumblingbiochemist.com/365-days-of-science/uv-light-what-happens-when-it-and-dna-or-rna-get-into-a-fight).
[33] ASHRAE. “Position Document on Filtration and Air Cleaning,” (www.ashrae.org/about/position-documents) (2021).
[34] CEC. “UL Report Air Quality Monitoring” (e425e57e-fa98-4fc2-a996-ae40a830082c.filesusr.com/ugd/e9ffe0_fed78a58964447df8a22bcdc090cd515.pdf) (August 20, 2020).
[35] CEC. “UL Remote Air Quality Monitoring” (e425e57e-fa98-4fc2-a996-ae40a830082c.filesusr.com/ugd/e9ffe0_fed78a58964447df8a22bcdc090cd515.pdf) (2020).
[36] “The Science Behind DHPTM (Dry Hydrogen Peroxide) Technology and the Synexis® BioDefense System” (synexis.com/wp-content/uploads/2020/12/The-Science-Behind-Dry-Hydrogen-Peroxide-Technology-and-the-Synexis®-BioDefense-System_1.pdf) (2020).
[37] Ramirez, et al. American Journal of Infectious Control; S0196-6553(20)30810-5 (doi.org/10.1016/j.ajic.2020.08.026) (2020).
[38] “How Plasmacluster ions are generated,” (global.sharp/pci/en/about_pci) (2021).
[39] Sharp Electronics. “Plasmacluster Ion inactivate Corona Virus” (press release: July 27, 2004) (sg.sharp/news/plasmacluster-ions-technology-inactivates-coronavirus).
[40] Sharp Electronics. “Plasmacluster*2 Ions Shown to Inhibit Infectivity of New-Type H1N1 Influenza Virus in Both Stationary and Airborne Form (press release: November 2, 2009) (global.sharp/pci/en/certified/pdf/viruses_02.pdf).
[41] Sharp Electronics. “Technology Inhibits Airborne Avian Influenza A (H7N9) Virus” (press release: November 11, 2015) (global.sharp/pci/en/certified/pdf/viruses_03.pdf).
[42] WHO. “Natural Ventilation for Infection Control in Health-Care Settings” (apps.who.int/iris/bitstream/handle/10665/44167/9789241547857_eng.pdf;jsessionid=71C9630ACAC17B142FF9A0773888C133?sequence=1) (2009).
[43] Healthline. “Breathing Rates” (www.healthline.com/health/normal-respiratory-rate#normal-rate-in-adults) (2021).
[44] O.H. Robertson, T.T. Puck, H.F. Lemon and G.L. Clayton. “The lethal effect of triethylene glycol vapor on air-borne bacteria and influenza virus”. Science 97 (2510): 142–144. (doi.org/10.1126/science.97.2510.142) (1943).
[45] Puck. “Mechanism of Aerial Disinfection by Glycols and other Chemical Agents” (www.ncbi.nlm.nih.gov/pmc/articles/PMC2135685/pdf/729.pdf) (1947).
[46] EPA. “EPA Approves Emergency Exemption for Antiviral Air Treatment” (www.epa.gov/newsreleases/epa-approves-emergency-exemption-antiviral-air-treatment) (2021).
[47] Grignard. “Air Treatment Science Study Funding Request” (2020).
[48] Amazon. “Triethylene Glycol” (www.amazon.com/Triethylene-Glycol-Possible-Containers-Resealable/dp/B085XK99VC).
[49] EPA. “Reregistration Eligibility Decision for Triethylene Glycol List C CASE 3146” (nepis.epa.gov) (2003).
[50] “Triethylene Glycol: Revised Antimicrobials Division’s Review of the Disciplinary Sciences for Issuance of the Reregistration Eligibility Decision (RED) document,” Reregistration Case No. 3146; PC Code 083501; CAS Registry No. 112-27-6; DP#: 305169 (www.regulations.gov/document?D=EPA-HQ-OPP-2005-0250-0002) (2006).
[51] Desai. “Grignard Pure NIOSH Air Sampling Triethylene Glycol concentration in the Air V6” (2020).
Get more of Elevator World. Sign up for our free e-newsletter.









